Analyzing Water in Oil
Tags: water in oil, oil analysis
High water content of lubricating oils negatively impacts the operation and longevity of the oils and the mechanical equipment components being lubricated. Water increases the oxidation rate of oils, thus prematurely using up the oils’ oxidation inhibitors. Additionally, water has been known to cause certain oil additives to precipitate out, as well as to chemically attack some additives.
Some of the modes by which water exists in lubricating oils can lead to catastrophic equipment failure. These include corrosion, erosion, etching and hydrogen embrittlement.
Water in oils can occur in dissolved, emulsified and free states. Visual indication is reliable for quantifying water content only in the free state, while the hot plate crackle test can be used to detect free and emulsified water.
However, neither of these methods can detect dissolved water or reproducibly detect trace levels of emulsified water. Furthermore, neither visual indication nor the hot plate test can be used to reliably quantify the water present. Distillation methods, such as ASTM D95 and D4006 provide better quantitative data in the range of approximately 500 ppm to 25 percent, but require large sample sizes and involve long analysis times, typically 60 to 110 minutes.
Since its invention by German petroleum chemist Dr. Karl Fischer in 1935, Karl Fischer (KF) analysis has progressed from an esoteric laboratory procedure to a widely accepted instrumental method routinely used for water determination in the petrochemical industry. It is estimated that nearly 500,000 KF determinations are performed daily around the world.
The method forms the basis of several commonly used ASTM standards for water determination in oils, including ASTM D1533, D1744, D4377, D4928 and D6304. The KF method does not suffer from the same issues and limitations associated with the other techniques described above, and a number of recent advances in titrator instrumentation and reagent formulations have further improved the accuracy and reproducibility of KF analyses.
Chemistry and Principles
Karl Fischer titration proceeds according to a reaction with a two-step mechanism in which sulfur dioxide initially reacts with an alcohol (ROH) to form an ester intermediate which is neutralized, or buffered, by an appropriate organic base (RN). The subsequent oxidation of the alkylsulfite salt to an alkylsulfate salt by iodine consumes water in a 1:1 ratio to iodine, thus making the quantification of water possible. The following reactions represent this two-step mechanism.
ROH + SO2 + RN → (RNH)·SO3R
(RNH).SO3R + 2 RN + I2 + H2O + → (RNH)·SO4R + 2 (RNH)I
The end-point determination in KF titration occurs by means of bivoltametric indication. That is, while the iodine in the Karl Fischer reagent is reacting with water, there is no free iodine present in the titration cell, and a high voltage is required to maintain the set polarization current at the double platinum pin indicator electrode.
Once all the water has reacted with the iodine, trace quantities of free iodine appear in the titration cell, causing a drop in voltage necessary to keep the polarization current constant, which in turn signals the end-point of the titration.
Types of Titration
Volumetric Karl Fischer
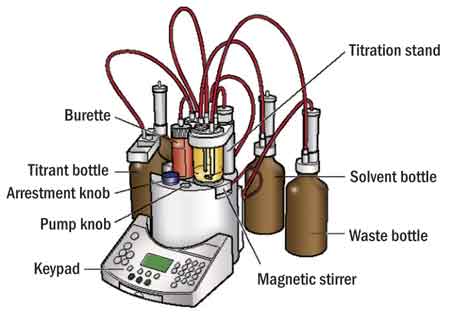
Volumetric Karl Fischer proceeds in the conventional manner of a classic titration, in that the titrant containing iodine is added mechanically to the solvent containing the sample by the titrator's burette (Figure 1).
The two types of volumetric KF differ in the exact composition of titrant and solvent. In one-component KF, the titrant (usually referred to as a CombiTitrant or a composite) contains all the ingredients needed for the KF reaction, namely iodine, sulfur dioxide, base and a suitable alcohol, while the solvent is typically dry methanol.
In two-component KF, the titrant contains only an alcoholic solution of iodine, while the solvent contains the other ingredients needed for the reaction. With both types of volumetric KF, imidazole is the base used most frequently as a buffer to maintain optimal pH for the reaction.
The most widely used standard methods based on volumetric KF are ASTM D1533 (Method A), D1744 and D4377. Volumetric KF is most accurate in the range of 500 ppm to 100 percent water.
Coulometric Karl Fischer
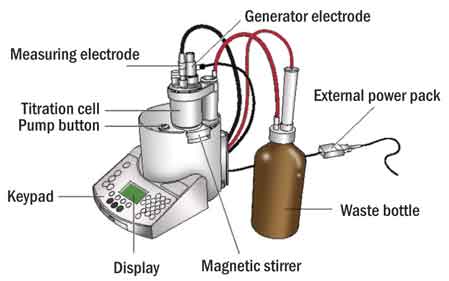
In Coulometric Karl Fischer, the iodine needed by the KF reaction is not present in the KF reagent, but is instead generated electrochemically in situ from iodide at the anode of the generator electrode, a component of the coulometric titration cell (Figure 2).
Corresponding reduction of hydride to hydrogen takes place at the cathode. In coulometry, the quantity of iodine generated corresponding to the amount of water present is calculated by the titrator on the basis of current (mA) and time (sec).
Coulometric Karl Fischer is considered an absolute method because time and current can both be accurately measured. The most widely used standard methods based on coulometric KF are ASTM D1533 (Method B), D4928 and D6304 (Method A). Coulometric KF is most accurate in the range of 1 ppm to 5 percent water.
Oil Sample Challenges
Solubility
If a sample does not dissolve fully during KF analysis, then only part of the water content will be determined, leading to erroneously low results. Oils have limited solubility in alcohols, such as methanol, which are typically used in common KF reagent formulations, while they are fully soluble in organic solvents like chloroform, toluene and xylene.
However, alcohols cannot be completely removed from KF reagents, because the presence of alcohol is required by the mechanism of the KF reaction.
Reactivity
Certain compounds in the oil will undergo interfering side reactions either with methanol or iodine components of KF reagents. A number of lubricating oil additives are reactive and are known to interfere with direct KF titration. These include aldehydes, higher phenols, modified mercaptans, ketoacids, polysiloxanes, sulfides and metal oxides.
Used oils may also contain contaminants that can potentially interfere with direct KF analysis, such as trace metals, polyaromatic hydrocarbons, polychlorinated biphenyls, as well as various products of incomplete combustion.
Sample Size and Reproducibility
As with any analytical technique, the sampling error in KF analysis is inversely proportional to the sample size. In other words, the larger the sample, the less error is introduced into the analysis by sample weighing, transfer and other handling. However, due to solubility and reactivity issues described above, analysts often drastically reduce sample size, which tends to reduce the reproducibility of test results.
Techniques for Successful KF of Oil Samples
Direct Titration
Sample solubility issues may be overcome by using co-solvents such as chloroform, toluene or xylene to increase the solvent capacity of conventional KF reagents in the titration cell. Alternatively, specially preformulated KF reagents incorporating one or several of these solvents are commercially available for both volumetric and coulometric KF.
For those oil samples which fail to adequately dissolve even when using KF reagents formulated with organic co-solvents, or those that are suspected of containing interfering compounds, an indirect KF analysis using an oil evaporator is recommended, as described below.

Indirect Titration Using an Oil Evaporator
The indirect titration method utilizes a specialty accessory, the oil evaporator, which is connected to a volumetric or coulometric KF titrator (Figure 3). This innovative KF technique is incorporated in ASTM D6304 (Method B) and is applicable to nearly all oil-based samples.
The procedure involves adding the oil sample to the solvent present in the evaporation chamber where it dissolves in the solvent, and in the process forms a binary azeotrope between the solvent and the sample's water content.
The solvent/sample mixture is then heated to near the azeotropic point and kept at that temperature while a dry, inert carrier gas, such as nitrogen, is used to carry the azeotropic vapor into the titration cell of the KF titrator, where the water content is quantified.
This method thus combines the best features of both Karl Fischer titration and distillation techniques, such as ASTM D95, because the oil evaporator is simply a miniaturized Dean & Stark distillation apparatus.
The distillation component of the indirect titration method ensures that the hard-to-dissolve oil samples, or those containing potentially interfering compounds, are not introduced to the titration cell, while the Karl Fischer titration component of the method accurately quantifies the water content of the azeotropic vapor carried into the titration cell by the dry gas.
Additionally, because the oil evaporator contains a miniaturized distillation set-up, only small sample (0.1 to 2.5g) and solvent (10 to 15mL) quantities are required.
Sample Size Selection
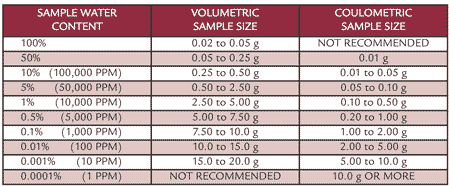
For both direct and indirect KF methods, sample size selection is critical for minimizing errors associated with sample weighing, transfer and injection. As a guideline, sample sizes shown in Table 1 are recommended for volumetric and coulometric Karl Fischer, respectively.
Karl Fischer analysis is a versatile and robust analytical tool for water content analysis in oil samples, and can detect water in any of three states commonly found in oils. The technique also offers other substantial advantages over more conventional hot plate crackle test and distillation methods, and it has been incorporated into numerous ASTM standards.
Volumetric or coulometric Karl Fischer methods, utilizing either direct titration or the oil evaporator technique, can be used to quantify water in oil samples from trace amounts to 100 percent.
References
- Slater, K. “Real-Time Monitoring of Water in Oil.” Lubrication Excellence 2005 Conference Proceedings. April 2005.
-
Duncanson, M. “Detecting and Controlling Water in Oil.” Practicing Oil Analysis magazine. September-October 2005.
-
Gebarin, S. “A Closer Look at Karl Fischer Coulometric Titration.” Practicing Oil Analysis magazine. March-April 2004.
-
Kunkel, S. and DeSandro, J. “Titration Method Improves Water Measurement.” Oil & Gas Journal. December 1986.
-
Rouessac, F. and Rouessac, A. Chemical Analysis: Modern Instrumental Methods and Techniques. (2000).
-
McClure, M. and Steffen, R. “Water Testing in Pharmaceutical Products.” Current Separations. August 2005.
- Mettler-Toledo GmbH. Application brochure 26. Fundamentals of Volumetric Karl Fischer Titration. 1999.